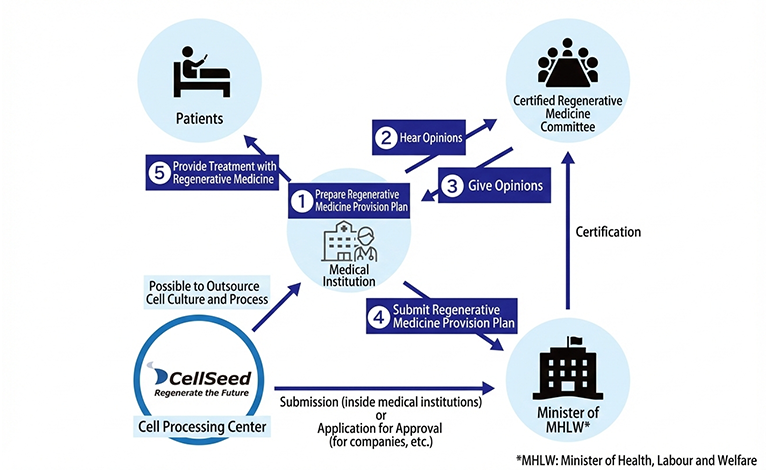
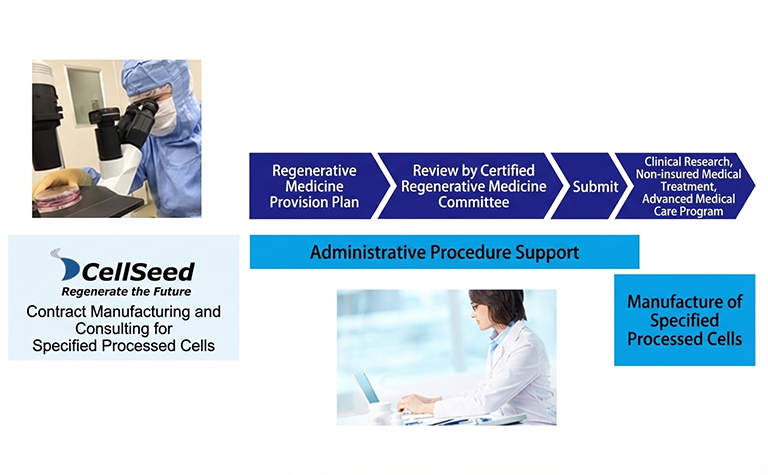
Contract Manufacturing and Consulting for Specified Cell-based Products
Our Cell Culture Center, which obtained a License for the Manufacturing of Specified Processed Cell Products in March 2017, manufactures specified processed cell products in compliance with the Act on the Safety of Regenerative Medicine. In addition, we provide comprehensive support for preparing application documents required for the provision of regenerative medicine, as well as assisting in the creation of documents necessary for operational management.
Inquiry
Please send your questions from below link