Contract Development and Manunfactiring Service
-
At our Cell Processing Facility, we engage in contracted business related to the manufacture of cell sheets, the operation of facilities, and the preparation of application documents. With a manufacturing and quality control system that conforms to Good Gene, Cellular, and Tissue-Based Products Manufacturing Practices(GCTP) in compliance with Japan's Act on the Safety of Regenerative Medicine, we provide safe and high quality products and services through our experienced staff.
In addition to contract manufacturing of cell sheets, we also provide clients with support needed to address and respond to authorities at each stage from product development and manufacturing through marketing; prepare applications for product approval; acquire approvals for manufacturing business and manufacturing / marketing business; and train technicians.
-

Features
-

-
1. Development of manufacturing methods for cell sheet products and contract manufacturing
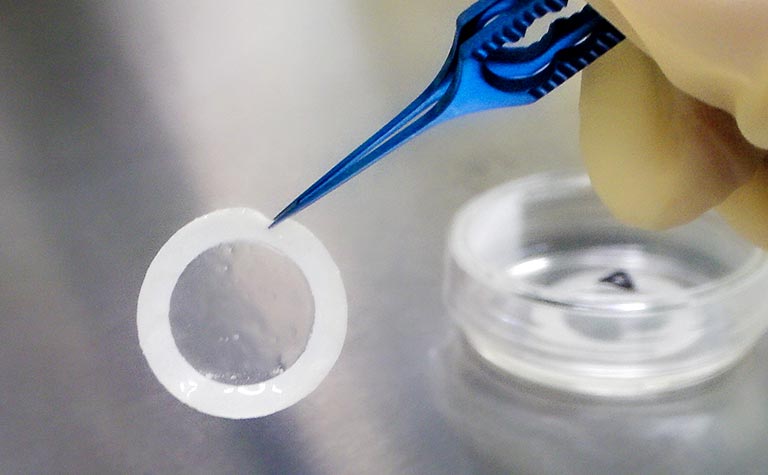
CellSeed provides services including development and manufacturing primarily of cell sheet products, upon receipt of contracted business from pharmaceutical companies and medical research institutions. The manufacturing methods developed and cell sheet products manufactured are of high quality, provided by cell culturing personnel who have been certified by the Japanese Society for Regenerative Medicine.
Services
- Contract manufacturing of cell sheet products
- Development of cell sheet manufacturing methods
- Quality management testing of cell sheets, etc.
-
2. Operational and Application support services
The Act on the Safety of Regenerative Medicine enforced in November 2014 stipulates the measures to be implemented by the service provider and the system for approval of the manufacturing processes for specified cell products. CellSeed provides various services to assist in obtainment of the license for the cell processing facility, and in the preparation of the necessary documents to submit for clinical trial notification, applications for government procedures, meetings with regulatory authorities, etc.
-

Services
- Preparation of application materials to be submitted to the Regulatory Authorities for obtaining license to manufacture specified cell products
- Creation of SOPs, Prescribed Rules, and other documentation
- Consulting
- Assistance with the maintenance and management of machinery and equipment in the Cell Processing Facility, and its management organization, etc.
-

-
3. Training and Education in cell culturing technology
In our Cell Processing Facility, training and education in the culturing and harvesting of cell sheets are provided, especially for less experienced technicians.
Service
- Cell sheet culturing training
- Cell sheet harvesting training, etc.
Inquiry
Contact us below about Contract Development and Manufacturing Service