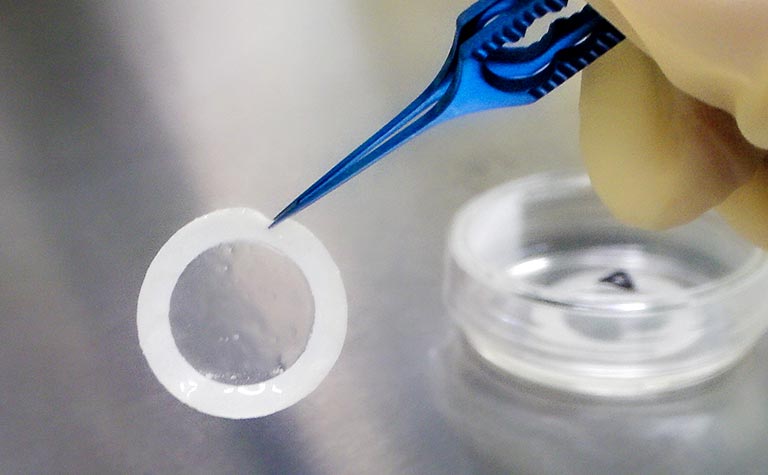
Contract Development and Manufacturing Service
-
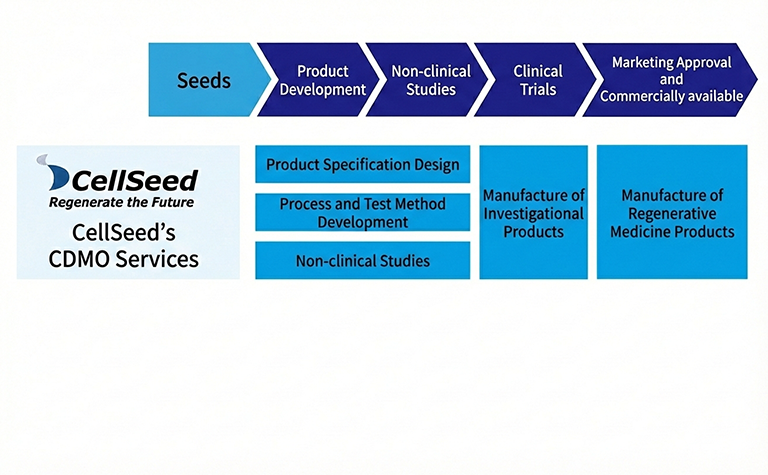
We proudly offer comprehensive services as a leading Contract Development and Manufacturing Organization (CDMO) for regenerative medicine products. At our state-of-the-art Cell Culture Center, which obtained a manufacturing license for regenerative medicine products in October 2018, we have established a robust manufacturing and quality control system fully compliant with the “Ministerial Ordinance on Standards for Manufacturing Control and Quality Control of Regenerative Medicine Products (GCTP Ordinance).” Leveraging advanced technologies and strict regulatory compliance, we deliver high-quality, reliable solutions to support the development and manufacturing of cutting-edge regenerative therapies.
-
-

-
CellSeed is listed in the “Regenerative Medicine Products CDMO Company List” prepared by the Ministry of Economy, Trade and Industry in cooperation with the industry association, the Regenerative Medicine Innovation Forum.
■Cdmo List | Forum for Innovative Regenerative Medicine
URL:https://firm.or.jp/en/cdmo/
Inquiry
Please send your questions from below link