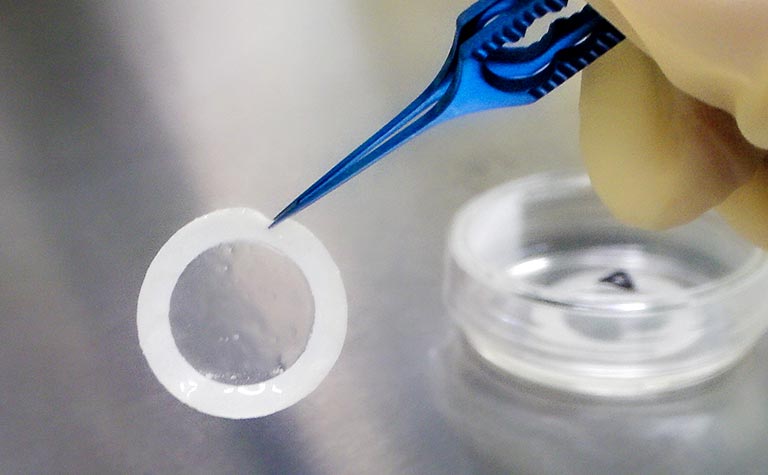
What's Regenerative Medicine
History of medicines and regenerative medicine
-
-
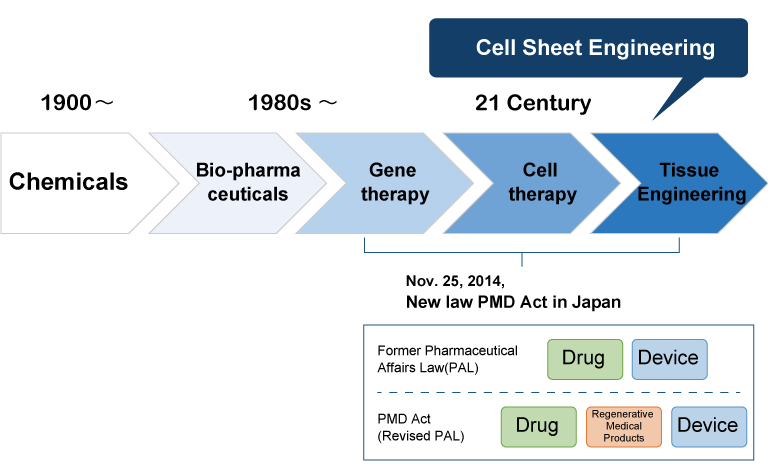
Most of the medicines we take today are made of chemicals.
As science progressed, the form of medicine changed, and there was a particularly big change in the 1980s. With gene recombination technology such as cutting out genes and transferring them to others, proteins that have human genes made in Escherichia coli have become drugs. Since then, research has been carried out using the gene itself as a drug, and further attempts are being made to administer cells and tissues as "drugs." This is called "regenerative medicine products".
In order to promote these efforts, the Government of Japan also revised the existing Pharmaceutical Affairs Law in 2014, "Act on Securing Quality, Effectiveness and Safety of Pharmaceuticals, Medical Devices, etc." and "Safety of Regenerative Medicine, etc." "Law on Securing, etc." was enacted. This has facilitated the development of new "drugs" that use cells and genes.
General new drug development process
-
-
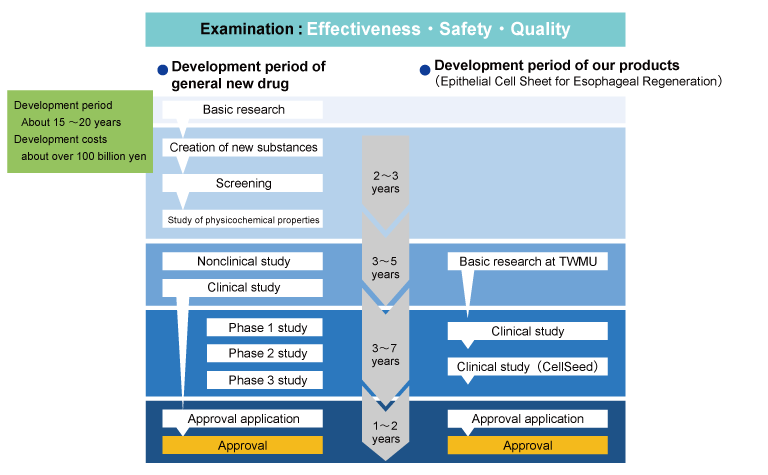
To develop a common new drug, first investigate the mechanism of the disease of interest. Then, a large number of chemical substances that are expected to be effective in curing the symptoms are synthesized, and screening is performed to select candidate chemical substances from them. After narrowing down the candidates for chemical substances, we will investigate the effects and safety using animals and cells. After confirming the efficacy and safety, we will first conduct a clinical trial (Phase 1 study) to confirm the safety in healthy people. Next, a clinical trial (Phase 2 study) will be conducted to confirm the efficacy of the drug by administering it to people with symptoms. Finally, we will conduct a large-scale clinical trial (Phase 3 study) in patients and healthy people. Phase 3 study are often a double-blind study, in which both the recipient and the recipient do not know whether it is a new drug or a placebo.
All of this data will be put together and submitted to the regulatory authorities of each country for approval, and only after approval is obtained will it be available as a product. There are three points to be examined: effectiveness, safety, and quality. It takes about 15 to 20 years from the development of a new drug to the medical field, and the development cost is increasing year by year, costing 100 billion yen or more.
The Act on the Safety of Regenerative Medicine and the Act on Pharmaceuticals and Medical Devices
-
-
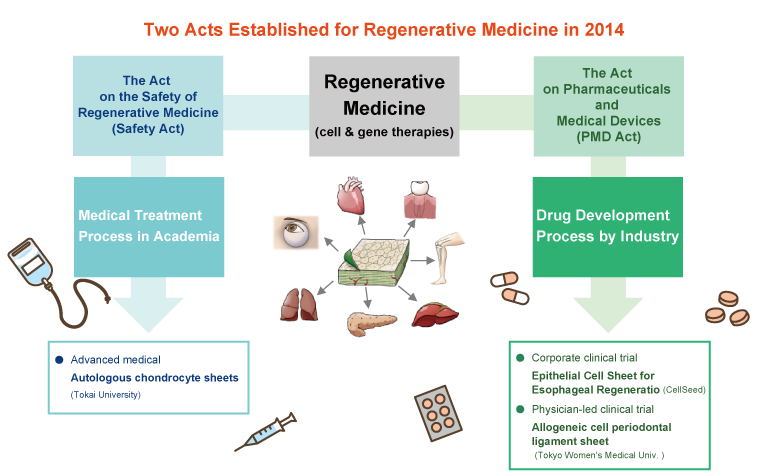
Regenerative medicine is conducted under two laws.
The Act on the Safety of Regenerative Medicine
It is a law that regulates regenerative medicine, etc., which is carried out under the responsibility of doctors.
This applies to clinical research and advanced medical care conducted at universities. Under this new law, cells used for treatment can be manufactured on a contract basis if the facility is recognized as having a quality assurance system and manufacturing capacity. Our cell culture center has obtained approval for a specific cell processing facility, and manufactures "autologous chondrocyte sheets" used in advanced medical care B conducted at Tokai University on a contract basis.The Act on Pharmaceuticals and Medical Devices
When a company obtains approval as a product, it is manufactured, sold, and approved under the Act on Pharmaceuticals and Medical Devices. The "allogeneic chondrocyte sheets" that we are currently aiming for early commercialization is carried out under this law.
Relationship between cell sheet and iPS cells
-
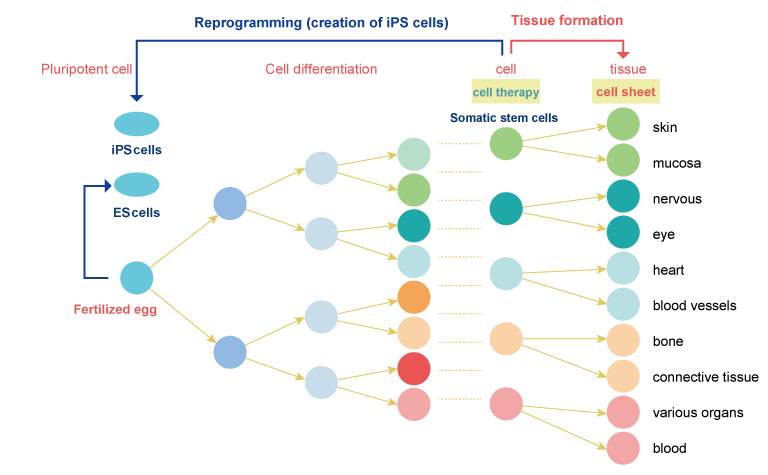
-
The smallest unit of our body is cells, and each body is made up of tens of trillions of cells. The cells are composed of cells with different appearances, shapes, and functions, such as skin, nerve cells, and heart. At first, it starts with only one cell and increases to one, two, four, and so on, and the shape and function are divided. Cell differentiation is the process of changing the shape and function of cells that carry the same gene as they increase. Until now, the differentiation of a fertilized egg was considered to be a one-way reaction. However, Yamanaka M.D., Ph.D. of Kyoto University succeeded in creating pluripotent cells that can differentiate into various functions and shapes similar to fertilized eggs by adding four genes to already differentiated skin cells. Yamanaka M.D., Ph.D. named this cell an iPS cell. Yamanaka M.D., Ph.D. received the Nobel Prize in Physiology or Medicine in 2014 for this research. Research is still underway on the complex mechanism by which cells with the same gene differentiate into various cells with different shapes and functions.
We are conducting research and development to sheet cells that are not iPS cells but somatic stem cells that are still capable of differentiating. The process by which iPS cells differentiate into the desired cells is very complex and still under research, so we use somatic stem cells and differentiated cells for faster commercialization. Cell sheets have the advantage that cells adhere to each other, proliferate in a state similar to cells in the body, and can be transplanted in a state where they can exert their original functions.
Regenerative medicine products approved in Japan
In Japan, as of April 2025, 20 items of regenerative medicine products have been approved.
6 items are tissue transplants, 5 items are cell transplants, 5 items are ex vivo gene therapy, 3 items are in vivo gene therapy, and 1 item is in vivo virus therapy.
| Company | Product | Target disease | Launch | |
|---|---|---|---|---|
| tissue transplants | J-TEC | JACE | Severe burns Congenital melanocytic nevus Epidermolysis bullosa |
2007 2016 2018 |
| J-TEC | JACC | Full-thickness articular cartilage defects of the knee due to trauma or osteochondritis dissecans Secondary knee osteoarthritis |
2012 2025 |
|
| J-TEC | Nepic | Corneal epithelial stem-cell deficiency Limbal Stem Cell Deficiency |
2020 | |
| J-TEC | Ocural | Corneal epithelial stem-cell deficiency Limbal Stem Cell Deficiency |
2021 | |
| Hirosaki Lifescience Innovation | Sakracy | Corneal epithelial stem-cell deficiency Limbal Stem Cell Deficiency |
2022 | |
| J-TEC | Jacemine | Stable vitiligo or Piebaldism | 2023 | |
| cell transplants | JCR Pharmaceuticals | TEMCELL HS | Acute graft-versus-host disease (GVHD) following hematopoietic stem cell transplant (GVHD) | 2015 |
| NIPRO | Stemirac | Improvement of neurologic symptoms and dysfunction associated with spinal cord injury | 2018 | |
| Takeda | Alofisel | Inactive or mildly active Crohn’s disease Complex anal fistula in a patient | 2021 | |
| Aurion Biotech | Vyznova | Bullous keratopathy | 2023 | |
| SanBio | AKUUGO | Traumatic spinal cord injury | 2024 | |
| ex vivo gene therapy (CAR-T) |
Novartis Pharma | Kymriah | Patients up to 25 years of age with B-cell precursor acute lymphoblastic leukemia (ALL) and Adult patients with relapsed or refractory (r/r) large B-cell lymphoma after two or more lines of systemic therapy including diffuse large B-cell lymphoma (DLBCL) | 2019 2022 |
| Daiichi-Sankyo | Yescarta | Adult patients with relapsed or refractory (r/r) large B-cell lymphoma after two or more lines of systemic therapy including diffuse large B-cell lymphoma (DLBCL) and Relapsed or Refractory Follicular Lymphoma and Marginal Zone Lymphoma | 2021 2022 |
|
| Bristol-Myers Squibb | Breyanji | Non-Hodgkin Lymphoma Diffuse Large B Cell Lymphoma Follicular Lymphoma Mantle-cell Lymphoma Primary Mediastinal B-cell Lymphoma |
2021 2022 |
|
| Bristol-Myers Squibb | Abecma | First-Anti-BCMA (B-cell Maturation Antigen) -CAR-T-Cell-Therapy-for-Relapsed-or-Refractory-Multiple-Myeloma/default.aspx | 2022 | |
| Janssen Pharmaceutical | Carvykti | Recurrent and refractory multiple myeloma | 2022 | |
| in vivo gene therapy |
Novartis Pharma | Zolgensma | Spinal muscular atrophy (SMA) with bi-allelic mutations in the survival motor neuron 1 (SMN1) gene | 2020 |
| Novartis Pharma | Luxturna | Inherited Retinal Dystrophy : IRD | 2023 | |
| Chugai | Elevidys | DMD : Duchenne muscular dystrophy | 2025 | |
| in vivo viral therapy |
Daiichi-Sankyo | Delytact | Malignant Glioma | 2021 |
FAQ
You can view frequently asked questions and answers regarding our business, including cell sheets.