Regenerative Medicine Contract Service
- Your trusted partner from compliance to manufacturing –
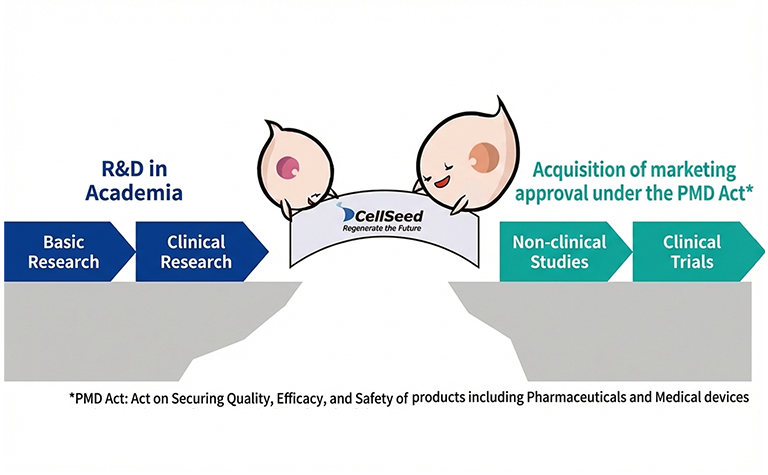
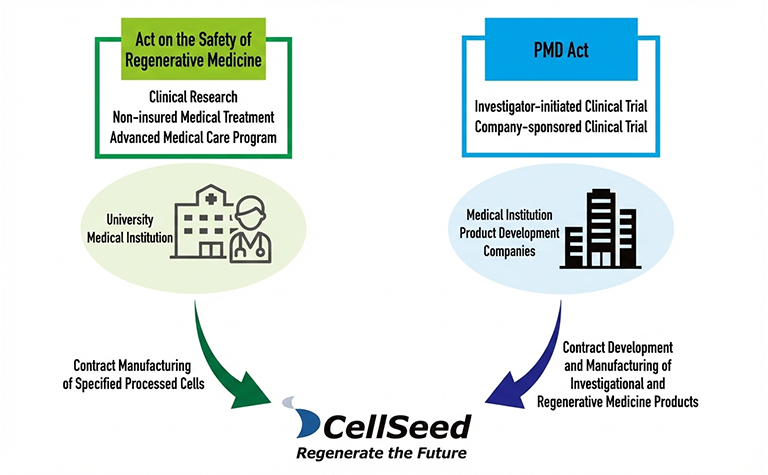
Leveraging our extensive experience in the development of regenerative medical products under the Act on Securing Quality, Efficacy and Safety of Pharmaceuticals and Medical Devices, as well as our proven track record in contract manufacturing of specified cell-processed products—including those used in Advanced Medical Care B—under the Act on the Safety of Regenerative Medicine, we provide solutions to bridge the gaps and overcome challenges faced by academia and industry.
Key Achievements
| Contract Manufacturing Products | Application | Cell Type | Indication | |
|---|---|---|---|---|
| Manufcturing Service | Cell sheet | Clinical Research | Autologous oral mucosal epithelial cells | Postoperatve treatment for congenital esophageal atresia (pediatric) |
| Cell sheet | Investigator-initiated clinical trial | Allogeneic periodontal ligament-delived MSCs | Periodontal tissue regeneration | |
| Cell sheet | Advanced Medical Care B program | Autologous Chondrocytes | Osteoarthritis of the knee | |
| Application Support | Cell sheet | Non-insured Medical Treatment | Autologous Chondrocytes | Osteoarthritis of the knee |
| Cell sheet | Non-insured Medical Treatment | Autologous oral mucosal epithelial cells | Prevention of post-ESD esophageal stenosis, refractory esophageal strictures |
Services
Inquiry
Please send your questions from below link