Allogeneic Chondrocyte Sheets
-
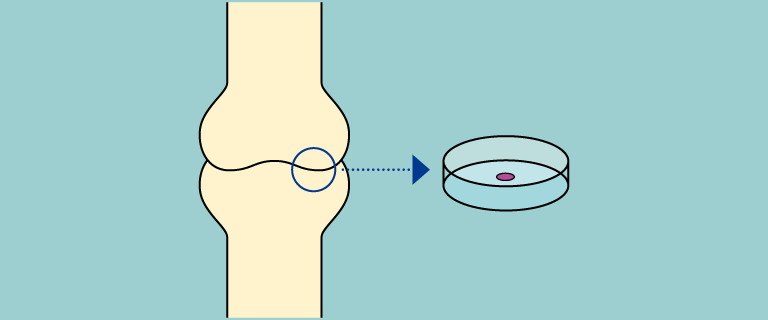
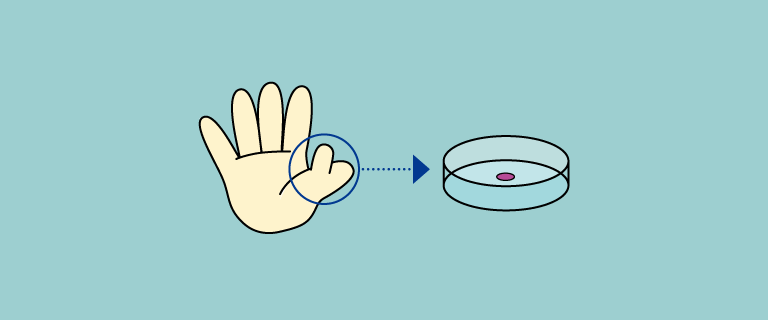
Allogeneic chondrocyte sheets are developed as a cell therapy product: chondrocytes are isolated from extra finger removed from patient with polydactyly, cultured for sheet fabrication, and transplanted to cartilage defect area.
This product is expected to reduce patient stress and treatment cost because patients can receive cell therapy without their own cartilage tissue collection.
Treatment method
-
-
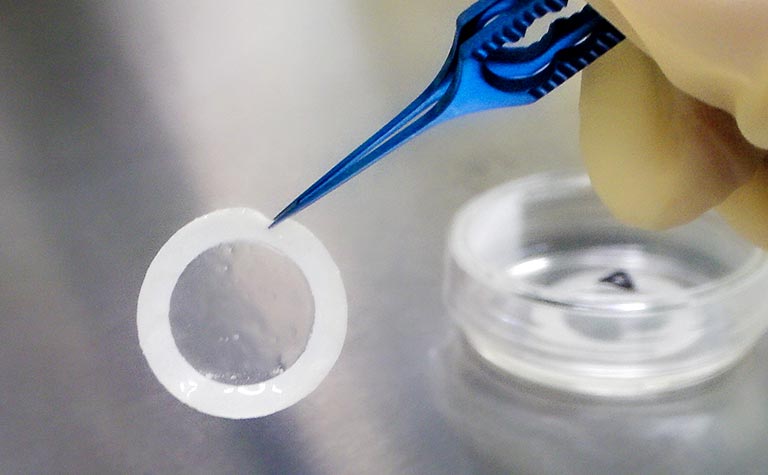
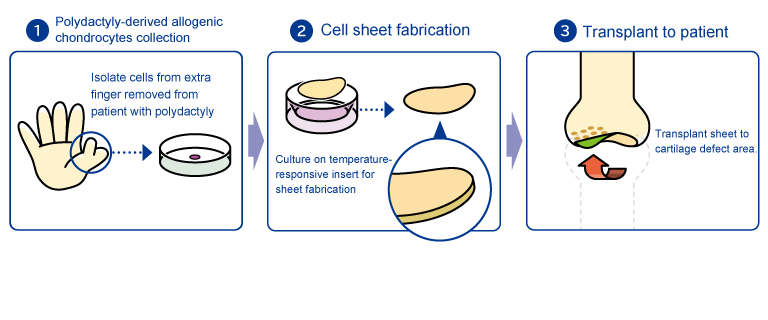
Chondrocytes are isolated from extra finger removed from patient with polydactyly.Cells cultured and seeded on temperature-responsive insert for sheet fabrication. Cell sheets are transplanted to the cartilage defect area.
Current Status of Autologous Chondrocyte Sheets
| 2017 - 2019 | 10 cases of transplantation were completed in the clinical research at Tokai University School of Medicine |
|---|---|
| 2018 - 2021 | Adopted as the ancillary project of AMED titled "Development of the evaluation methods for the efficacy of allogenic chondrocyte sheets)”; project period: Oct. 2018 to Mar. 2021 |
| 2020 | Tissue (for commercial purposes) supply from National Center for Child Health and Development has been commenced |
| 2021 - 2023 | Adopted as the ancillary project of AMED titled “Research and development for starting the clinical trial by industry, including the establishment of a cell bank for the commercialization of an allogeneic chondrocyte sheet (CLS2901C)”; project period: Aug. 2021 to Mar. 2023 |
| End of 2022 | Clinical trial notification submission (scheduled) in Japan |
Cell Sheet Regenerative Medicine Business
Chondrocyte Sheet
Inquiry
Contact us below about Cell Sheet Regenerative Medicine Business